- Home
- Steam Resources
- Steam Theory
- Comparing Steam and Hot Water Heating
Steam Control
Comparing Steam and Hot Water Heating
Differences between Steam and Hot Water Heating
Both steam and hot water are commonly used as heating media in industrial applications. They may look different, but both are, in fact, water. So, how should you use these two heat sources differently?
At atmospheric pressure, water boils at 100°C [212°F]. To use liquid water at temperatures above 100°C, you must continuously apply pressure above the saturation level to the system.
Conversely, since the saturation temperature of steam at atmospheric pressure is 100°C, it is not possible to create saturated steam below 100°C simply by reducing its pressure with a pressure reducing valve. A vacuum pump is necessary to precisely control steam at below 100°C.
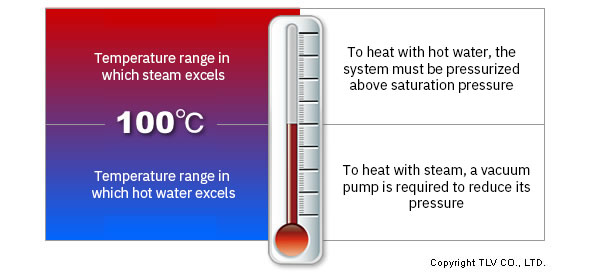
Because of this, in the majority of cases, hot water is used to heat at less than 100°C and steam is used for heating at over 100°C.
How Saturated Steam and Hot Water Transfer Heat
Herein, we will refer to saturated steam simply as steam. As a heating medium, steam offers the following advantages:
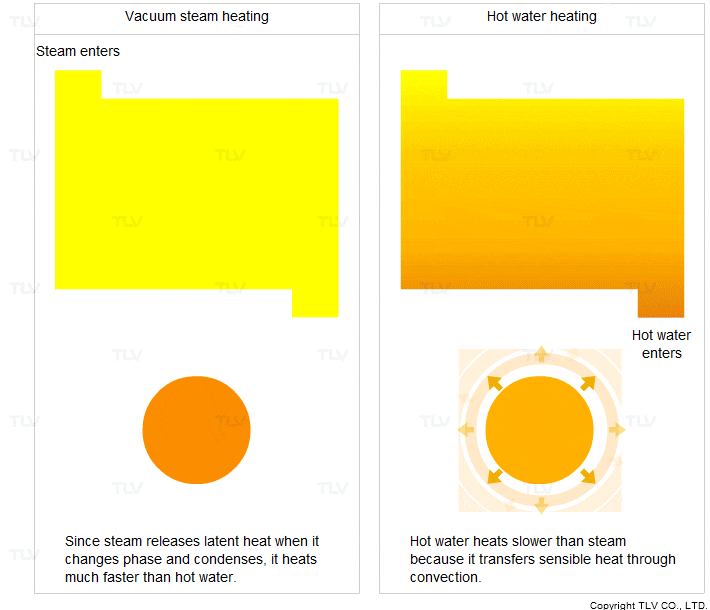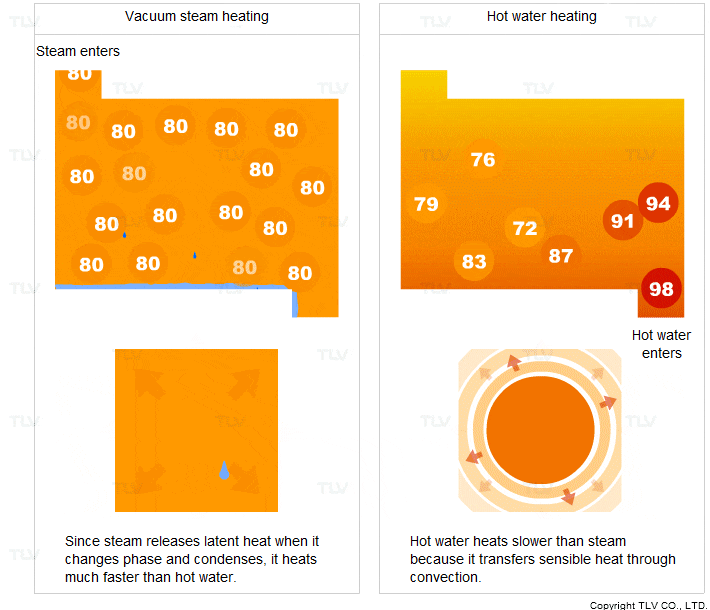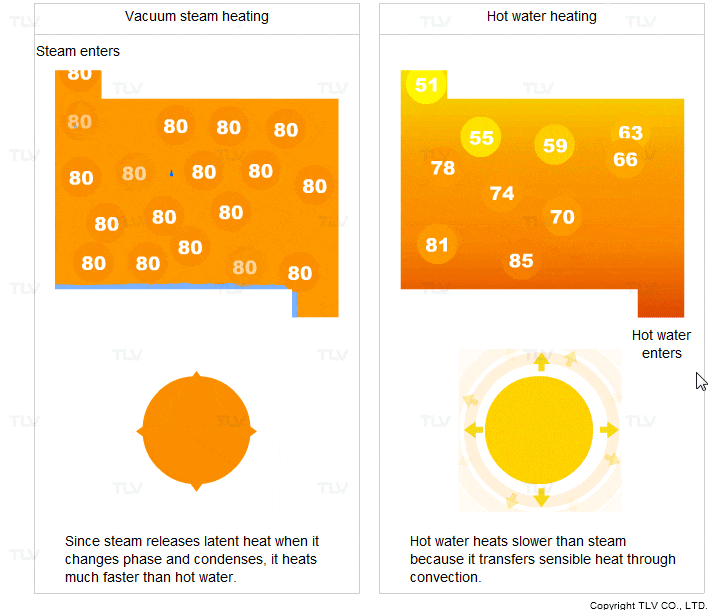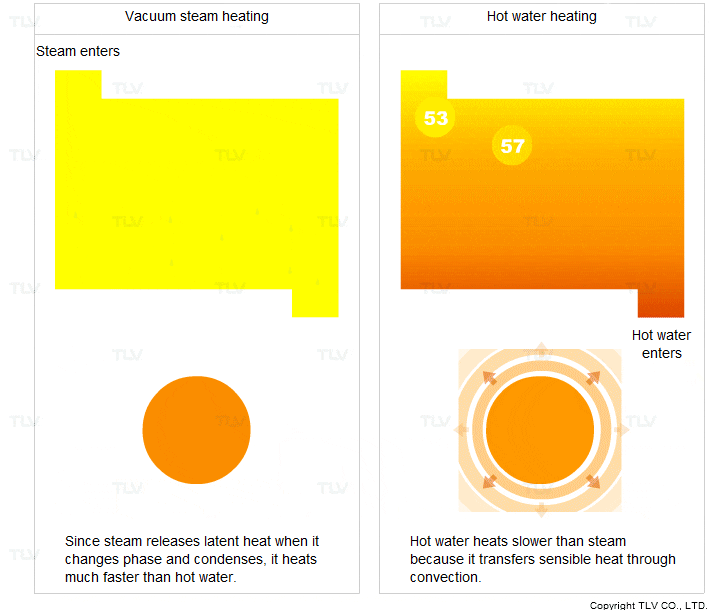
- It transfers heat through condensation. When it condenses, it releases a large amount of latent heat.
- It condenses at a constant temperature (the saturation temperature).
For these two reasons, steam heats faster and more evenly compared to hot water.
Vacuum steam heating systems have been developed so that the advantages of saturated steam can be utilized for heating below 100°C. In contrast, hot water transfers sensible heat through convection. In this process, the hot water transfers its heat to the product and its own temperature drops.
Hot water heating is therefore less uniform and transfers heat slower compared to steam heating.
Applications Where Hot Water Excels
With the advent of vacuum steam heating systems, steam heating has become possible even below 100°C. Is there still any merit in hot water heating, which is inferior to steam heating in terms of uniformity and heat transfer rate?
We argue that there is. Hot water heating has different features from steam heating. There are some heating processes in which the product itself generates heat (for example through an exothermic reaction) and becomes hotter than the heating medium. If this additional heat is not removed properly, the overall temperature may rise too high and the product may be ruined.
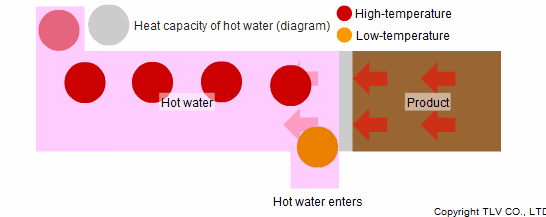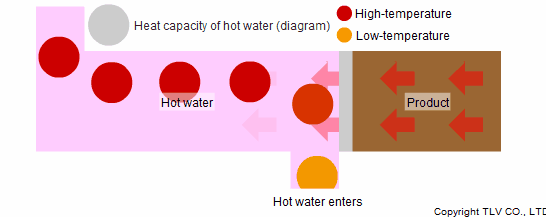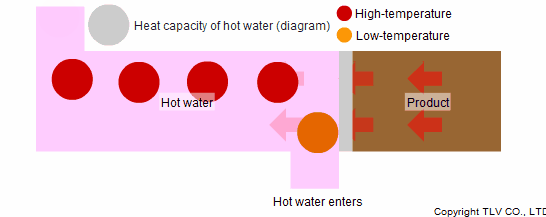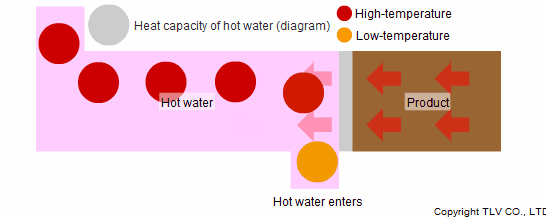
To prevent this, the excess heat needs to be removed and the product must be properly cooled. Hot water is a powerful tool in such a case. Hot water can be used as a medium for cooling products. In cooling processes, hot water absorbs the heat of the product again through convection. It collects this heat with the same heat transfer efficiency as when imparting it in heating processes.
Such processes employ forced convection, a heat transfer method in which hot water is constantly passed across the heat transfer surface. Through forced convection, the water absorbs the heat of the product making it possible to cool the system by transferring heat to the outside.
Applications Best Suited to Each Medium
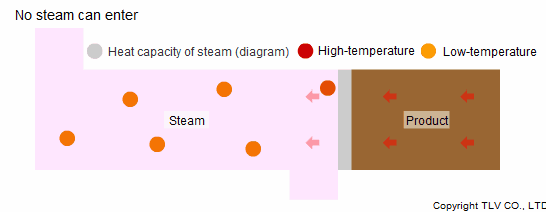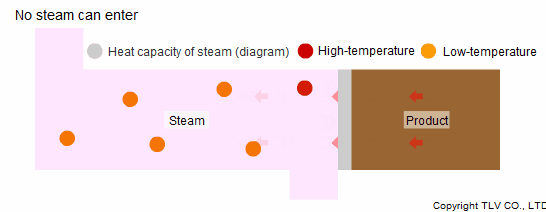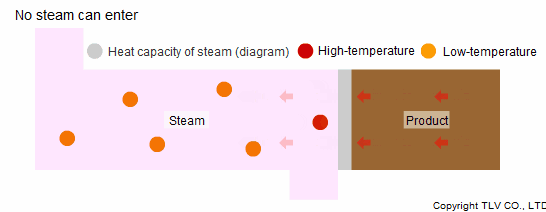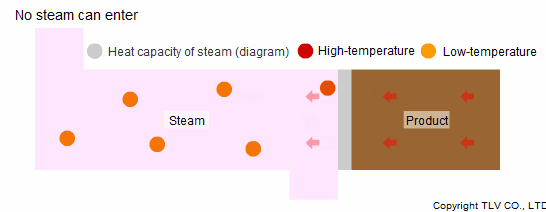
What if steam were used in a cooling application? Like with hot water, when steam is cooler than the product, it absorbs some of the product's heat as it moves across the heat transfer surface.
When steam is used in a heating process, it condenses after it loses its heat, allowing new steam to enter the chamber. However, when steam itself is heated, it does not condense, and so new steam cannot flow into the chamber. Furthermore, since a steam trap should be preventing it from flowing out of the equipment, the steam cannot take enough heat away from the product to cool it.
Another reason why steam is not suited to indirect cooling processes is that its heat capacity is smaller than that of hot water.
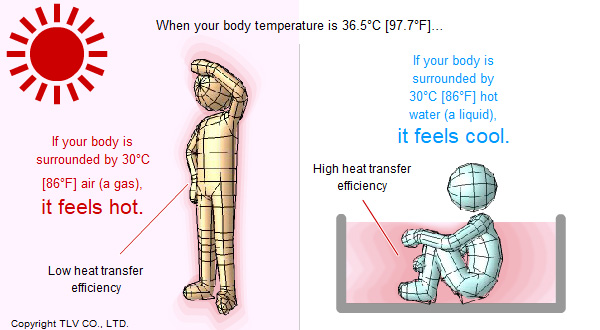
For example, for adult humans, who typically have a body temperature of around 36.5°C [97.7°F], standing outside on a 30°C [86°F] day feels hot, but sitting in a 30°C bath water can give you a chill. Even at the same temperature, air (a gas like steam) will absorb less heat from the human body than water due to its lower heat transfer efficiency.
- Steam heating: Quick and uniform heat transfer, useful in heating processes
- Hot water heating: Capable of both heating and cooling, advantageous in heating processes that involve cooling
Keep these differences in mind when choosing whether to heat with steam or hot water.
When it comes to cooling processes, however, there is another method that should be considered as an alternative to hot water: vacuum steam cooling. We discuss vacuum steam cooling in more detail in this article.

