- Home
- Steam Resources
- Steam Theory
- How to Read a Steam Table
Basics of Steam
How to Read a Steam Table
Just as a map (or GPS navigation system) is necessary when driving in a new area or a flight timetable is indispensable when taking the plane, steam tables are essential to steam users in industry. This article will introduce steam tables, pointing out the different types and offering an overview of the different elements found within them.
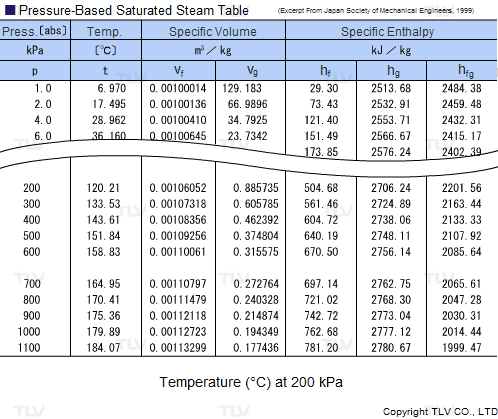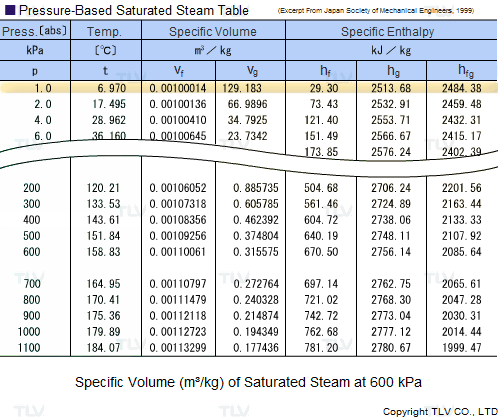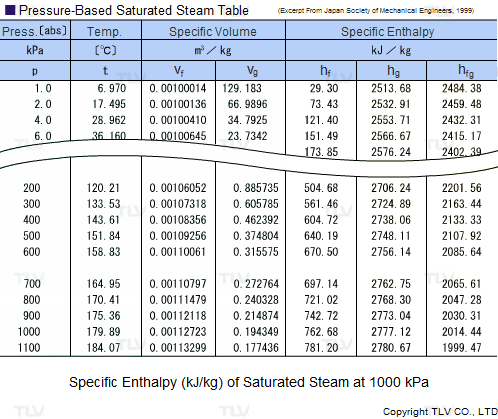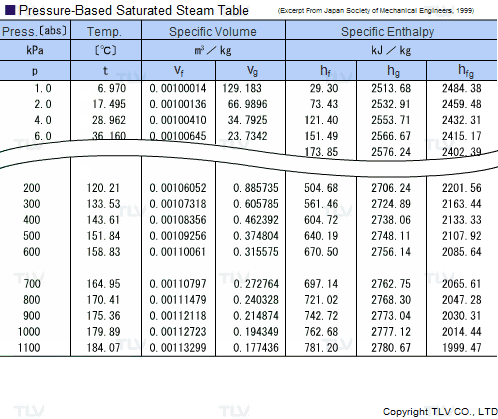
Saturated Steam Tables
A saturated steam table is an indispensable tool for any engineer working with steam. It's typically used to determine saturated steam temperature from steam pressure, or the opposite: pressure from saturated steam temperature. In addition to pressure and temperature, these tables usually include other related values such as specific enthalpy (h) and specific volume (v).
The data found in a saturated steam table always refers to steam at a particular saturation point, also known as the boiling point. This is the point where water (liquid) and steam (gas) can coexist at the same temperature and pressure. Because H2O can be either liquid or gas at its saturation point, two sets of data are required: data for saturated water (liquid), which is typically marked with an "f" in subscript, and data for saturated steam (gas), which is typically marked using a "g" in subscript.
| Example of Saturated Steam Table |
|---|
|
Legend:
|
Heating processes using steam generally use the latent heat of evaporation (Hfg) to heat the product. As seen in the table, this latent heat of evaporation is greatest at lower pressures. As saturated steam pressure rises, the latent heat of evaporation gradually decreases until it reaches 0 at supercritical pressure, i.e. 22.06 MPa (3200 psi).
Tip
Looking for TLV's online steam tables?
Access them here:
Two Formats: Pressure Based and Temperature Based
Since saturated steam pressure and saturated steam temperature are directly related to one another, saturated steam tables are generally available in two different formats: based on pressure and based on temperature. Both types contain the same data that is simply sorted differently.
Pressure Based Saturated Steam Table
| Press. (Gauge) | Temp. | Specific Volume | Specific Enthalpy | |||
|---|---|---|---|---|---|---|
| kPaG | °C | m3/kg | kJ/kg | |||
| P | T | Vf | Vg | Hf | Hfg | Hg |
| 0 | 99.97 | 0.0010434 | 1.673 | 419.0 | 2257 | 2676 |
| 20 | 105.10 | 0.0010475 | 1.414 | 440.6 | 2243 | 2684 |
| 50 | 111.61 | 0.0010529 | 1.150 | 468.2 | 2225 | 2694 |
| 100 | 120.42 | 0.0010607 | 0.8803 | 505.6 | 2201 | 2707 |
Temperature Based Saturated Steam Table
| Temp. | Press. (Gauge) | Specific Volume | Specific Enthalpy | |||
|---|---|---|---|---|---|---|
| °C | kPaG | m3/kg | kJ/kg | |||
| T | P | Vf | Vg | Hf | Hfg | Hg |
| 100 | 0.093 | 0.0010435 | 1.672 | 419.1 | 2256 | 2676 |
| 110 | 42.051 | 0.0010516 | 1.209 | 461.4 | 2230 | 2691 |
| 120 | 97.340 | 0.0010603 | 0.8913 | 503.8 | 2202 | 2706 |
| 130 | 168.93 | 0.0010697 | 0.6681 | 546.4 | 2174 | 2720 |
| 140 | 260.18 | 0.0010798 | 0.5085 | 589.2 | 2144 | 2733 |
| 150 | 374.78 | 0.0010905 | 0.39250 | 632.3 | 2114 | 2746 |
Different Units: Gauge Pressure and Absolute Pressure
Saturated steam tables can also use two different types of pressure: absolute pressure and gauge pressure.
- Absolute pressure is zero-referenced against a perfect vacuum.
- Gauge pressure is zero-referenced against atmospheric pressure (101.3 kPa, or 14.7 psi).
Saturated Steam Table using Absolute Pressure
| Press (Abs.) | Temp. | Specific Volume | Specific Enthalpy | |||
|---|---|---|---|---|---|---|
| kPa | °C | m3/kg | kJ/kg | |||
| P | T | Vf | Vg | Hf | Hfg | Hg |
| 0 | -- | -- | -- | -- | -- | -- |
| 20 | 60.06 | 0.0010103 | 7.648 | 251.4 | 2358 | 2609 |
| 50 | 81.32 | 0.0010299 | 3.240 | 340.5 | 2305 | 2645 |
| 100 | 99.61 | 0.0010432 | 1.694 | 417.4 | 2258 | 2675 |
Saturated Steam Table using Gauge Pressure
| Press. (Gauge) | Temp. | Specific Volume | Specific Enthalpy | |||
|---|---|---|---|---|---|---|
| kPaG | °C | m3/kg | kJ/kg | |||
| P | T | Vf | Vg | Hf | Hfg | Hg |
| 0 | 99.97 | 0.0010434 | 1.673 | 419.0 | 2257 | 2676 |
| 20 | 105.10 | 0.0010475 | 1.414 | 440.6 | 2243 | 2684 |
| 50 | 111.61 | 0.0010529 | 1.150 | 468.2 | 2225 | 2694 |
| 100 | 120.42 | 0.0010607 | 0.8803 | 505.6 | 2201 | 2707 |
Gauge pressure was created because it is often easier to reference measured pressure against the pressure we normally experience.
Steam tables based on gauge pressure indicate atmospheric pressure as 0, while steam tables based on absolute pressure indicate it as 101.3 kPa (14.7 psi). Also, to distinguish gauge pressure from absolute pressure, a "g" is typically added to the end of the pressure unit, for example kPaG or psig.
| Converting Gauge Units to Absolute Units |
|---|
For SI UnitsSteam Pressure [kPa abs] = Steam Pressure [kPaG] + 101.3 kPa For Imperial UnitsSteam Pressure [psi abs] = Steam Pressure [psiG] + 14.7 psi |
Important note: Problems can easily occur when absolute pressure is mistaken for gauge pressure (or vice versa), so it is always extremely important to pay close attention to the pressure units used in the table.
| Summary Table |
|---|
Gauge pressure:
Absolute pressure:
|
*Atmospheric pressure is 101.3 kPa (14.7 psi)
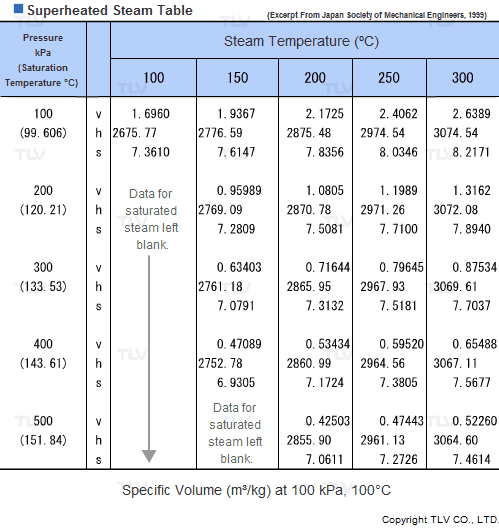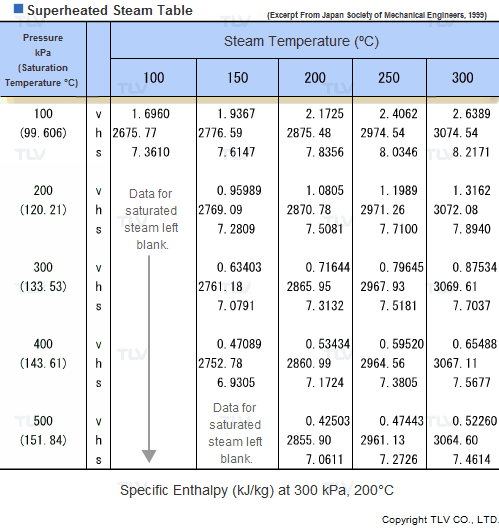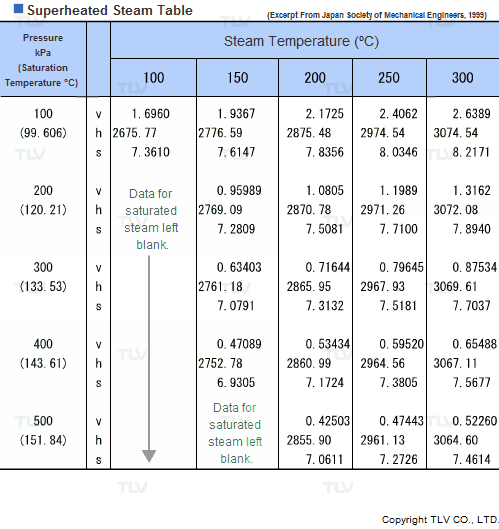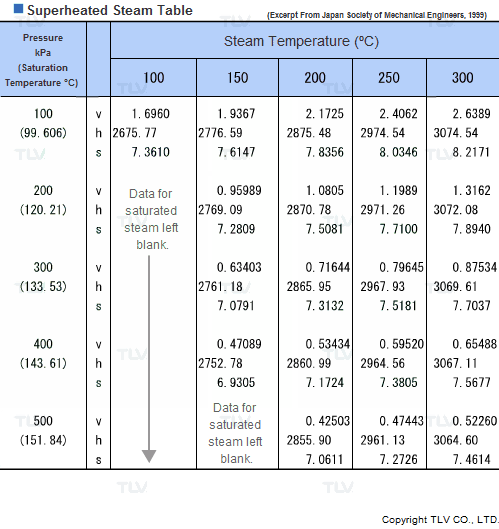
Superheated Steam Tables
Values related to superheated steam cannot be obtained through a regular saturated steam table, but rather require the use of a Superheated Steam Table. This is because the temperature of superheated steam, unlike saturated steam, can vary considerably for a same pressure.
In fact, the number of possible temperature-pressure combinations is so great that it would be virtually impossible to gather them all in a single table. As a result, a large number of superheated steam tables use representative pressure-temperature values to form a summary table.
| Example of Superheated Steam Table |
|---|
|
| The above superheated steam table contains data about Specific Volume (Vg), Specific Enthalpy (Hg) and Specific Heat (Sg) at typical values of pressure and temperature. |