- Home
- Steam Resources
- Steam Theory
- Types of Steam
Basics of Steam
Types of Steam
If water is heated beyond the boiling point, it vaporizes into steam, or water in the gaseous state. However, not all steam is the same. The properties of steam vary greatly depending on the pressure and temperature to which it is subject.
In the article Principal Applications for Steam, we discussed several applications in which steam is used. In the sections that follow, we will discuss the types of steam used in these applications.
Pressure-Temperature Relationship of Water & Steam
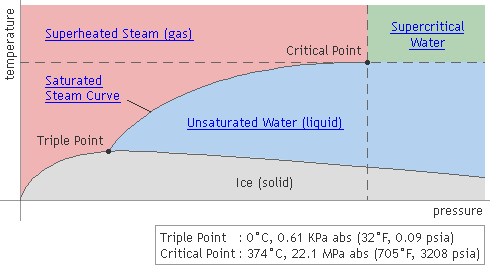
Saturated (dry) steam results when water is heated to the boiling point (sensible heating) and then vaporized with additional heat (latent heating). If this steam is then further heated above the saturation point, it becomes superheated steam (sensible heating).
Saturated Steam (Dry)
As indicated by the black line in the above graph, saturated steam occurs at temperatures and pressures where steam (gas) and water (liquid) can coexist. In other words, it occurs when the rate of water vaporization is equal to the rate of condensation.
Advantages of using saturated steam for heating
Saturated steam has many properties that make it an excellent heat source, particularly at temperatures of 100 °C (212°F) and higher. Some of these are:
| Property | Advantage |
| Rapid, even heating through latent heat transfer | Improved product quality and productivity |
| Pressure can control temperature | Temperature can be quickly and precisely established |
| High heat transfer coefficient | Smaller required heat transfer surface area, enabling reduced initial equipment outlay |
| Originates from water | Safe, clean, and low-cost |
Tip
Having said this, it is necessary to be mindful of the following when heating with saturated steam:
- Heating efficiency may be diminished if steam other than dry steam is used for process heating. Contrary to common perception, virtually all of the steam generated from a boiler is not dry saturated steam, but wet steam, which contains some non-vaporized water molecules.
- Radiant heat loss causes some of the steam to condense. The generated wet steam thus becomes even more wet, and condensate also forms, which must be removed by installing steam traps at appropriate locations.
- Heavy condensate that falls out of the steam flow can be removed through drip leg steam traps. However, the entrained wet steam will reduce heating efficiency, and should be removed through point-of-use or distribution separation stations
- Steam that incurs pressure losses due to piping friction, etc., may result a corresponding loss in steam temperature as well
Unsaturated Steam (Wet)
This is the most common form of steam actually experienced by most plants. When steam is generated using a boiler, it usually contains wetness from non-vaporized water molecules that are carried over into the distributed steam. Even the best boilers may discharge steam containing 3% to 5% wetness. As the water approaches the saturation state and begins to vaporize, some water, usually in the form of mist or droplets, is entrained in the rising steam and distributed downstream. This is one of the key reasons why separation is used to dis-entrain condensate from distributed steam.
Superheated Steam
Superheated steam is created by further heating wet or saturated steam beyond the saturated steam point. This yields steam that has a higher temperature and lower density than saturated steam at the same pressure. Superheated steam is mainly used in propulsion/drive applications such as turbines, and is not typically used for heat transfer applications.
Advantages of using superheated steam to drive turbines:
- Advantages of using superheated steam to drive turbines:
To maintain the dryness of the steam for steam-driven equipment, whose performance is impaired by the presence of condensate - To improve thermal efficiency and work capability, e.g. to achieve larger changes in specific volume from the superheated state to lower pressures, even vacuum.
It is advantageous to both supply and discharge the steam while in the superheated state because condensate will not be generated inside steam-driven equipment during normal operation, minimizing the risk of damage from erosion or carbonic acid corrosion. In addition, as the theoretical thermal efficiency of the turbine is calculated from the value of the enthalpy at the turbine inlet and outlet, increasing the degree of superheating as well as the pressure raises the enthalpy at the turbine inlet side, and is thereby effective at improving thermal efficiency.
Disadvantages of using superheated steam for heating:
| Property | Disadvantage |
| Low heat transfer coefficient | Reduced productivity |
| Larger heat transfer surface area needed | |
| Variable steam temperature even at constant pressure | Superheated steam needs to maintain a high velocity, otherwise the temperature will drop as heat is lost from the system |
| Sensible heat used to transfer heat | Temperature drops can have a negative impact on product quality |
| Temperature may be extremely high | Stronger materials of construction may be needed, requiring higher initial equipment outlay |
For these reasons and others, saturated steam is preferred over superheated steam as the heating medium in exchangers and other heat transfer equipment. On the other hand, when viewed as a heat source for direct heating as a high temperature gas, it has an advantage over hot air in that it can be used as a heat source for heating under oxygen-free conditions. Research is also being carried out on the use of superheated steam in food processing applications such as cooking and drying.
Supercritical Water
Supercritical water is water in a state that exceeds its critical point: 22.1MPa, 374 °C (3208 psia, 705°F). At the critical point, the latent heat of steam is zero, and its specific volume is exactly the same whether considered liquid or gaseous. In other words, water that is at a higher pressure and temperature than the critical point is in an indistinguishable state that is neither liquid nor gas.
Supercritical water is used to drive turbines in power plants which demand higher efficiency. Research on supercritical water is being performed with an emphasis on its use as a fluid that has the properties of both a liquid and a gas, and in particular on its suitability as a solvent for chemical reactions.
Various States of Water
Unsaturated Water
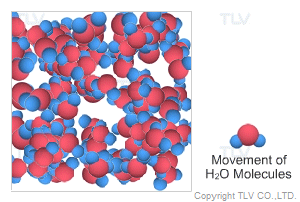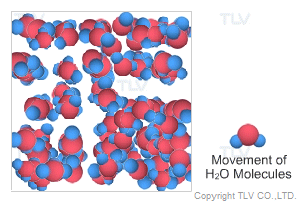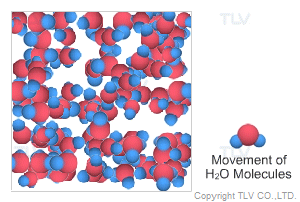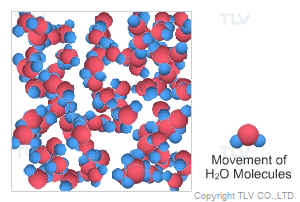
This is water in its most recognizable state. Approximately 70% of the weight of the human body is from water. In water's liquid form, hydrogen bonding pulls water molecules together. As a result, unsaturated water has a relatively compact, dense, and stable structure.
Saturated Steam

Saturated steam molecules are invisible. When saturated steam is released to the atmosphere by being vented from piping, part of it condenses by transferring its heat to the surrounding air, and clouds of white vapor (tiny droplets of water) are formed. When steam includes these tiny droplets, it is called wet steam.
In a steam system, steam released from steam traps is often misinterpreted to be saturated (live) steam, while it is in fact flash steam. The difference between the two is that saturated steam is invisible immediately at the outlet of the pipe whereas flash steam contains visible water droplets the instant it is formed.
Superheated Steam

As long as it retains its superheated state, superheated steam will not condense even if it comes into contact with the atmosphere and its temperature drops. As a result, no clouds of vapor are formed. Superheated steam stores more heat than does saturated steam at the same pressure, and the movement of its molecules is more rapid so it is has lower density (i.e., its specific volume is greater).
Supercritical Water
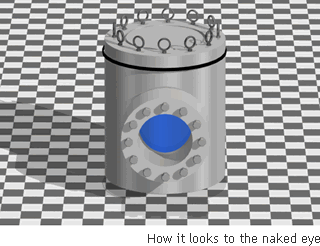
Though it is not possible to tell by visual observation, this is water in a form that is neither liquid nor gaseous. The general idea is of a molecular movement that is close to that of gas, and a density that is closer to that of a liquid.

