- Home
- Steam Resources
- Steam Theory
- Wet Steam vs. Dry Steam: The Importance of the Steam Dryness Fraction
Basics of Steam
Wet Steam vs. Dry Steam: The Importance of the Steam Dryness Fraction
Did you know that boilers do not generate 100% saturated steam (dry steam)? When a steam boiler heats up water, bubbles breaking through the water surface will pull tiny water droplets in with the steam. Unless a superheater is used, this will cause the steam supply to become partially wet (wet steam) from the added liquid.
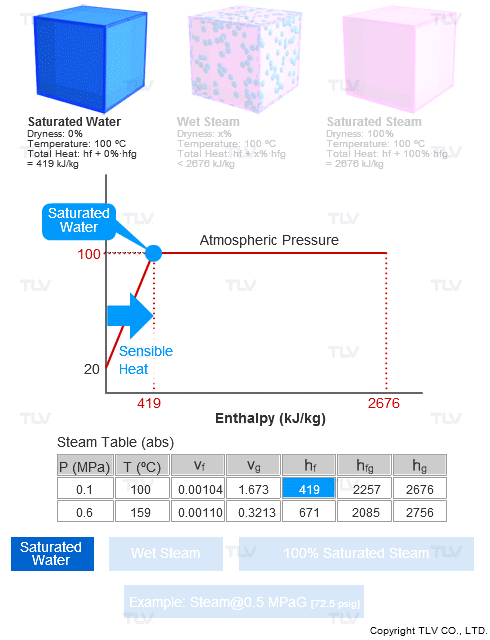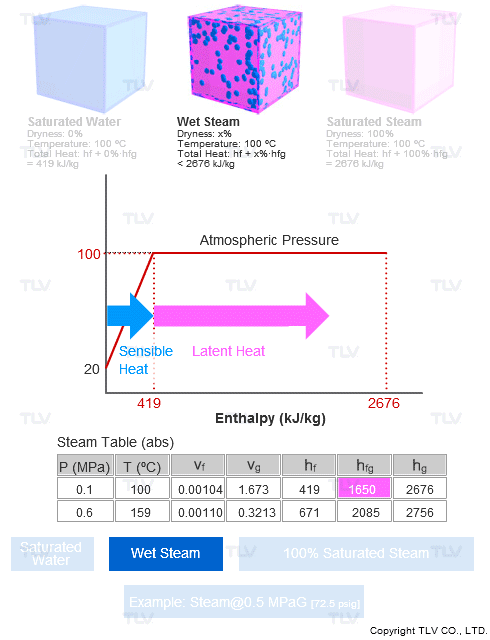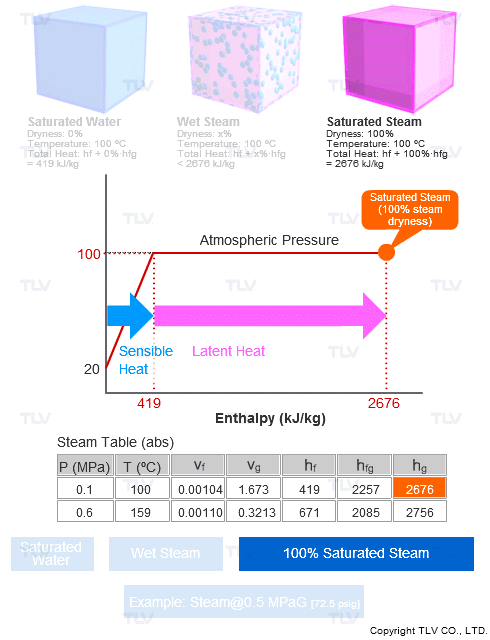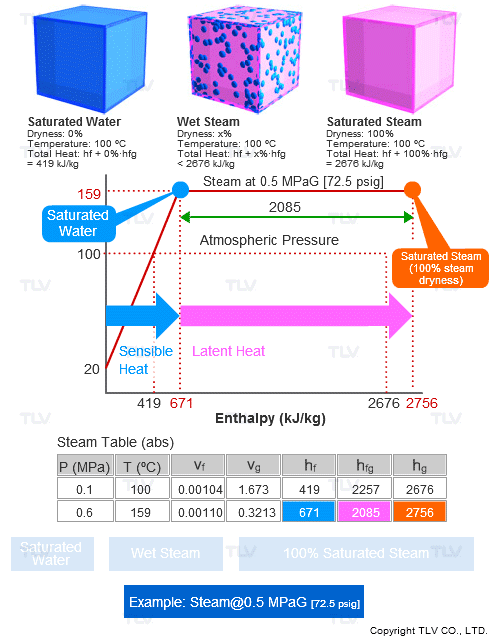
Steam Dryness Fraction
The steam dryness fraction is used to quantify the amount of water within steam. If steam contains 10% water by mass, it's said to be 90% dry, or have a dryness fraction of 0.9.
Steam dryness is important because it has a direct effect on the total amount of transferable energy contained within the steam (usually just latent heat), which affects heating efficiency and quality.
For example, saturated steam (100% dry) contains 100% of the latent heat available at that pressure. Saturated water, which has no latent heat and therefore 0% dryness, will only contain sensible heat.
Steam Dryness = 100% - [% Entrained Water] (by mass)
Calculating the Total Heat of Wet Steam
Steam tables contain values such as enthalpy (h), specific volume (ν), entropy (s), etc. for saturated steam (100% dry) and for saturated water (0% dryness), but typically not for wet steam.
These can be calculated by simply considering the ratio of steam to water, as described in the equations below:
Specific Volume (ν) of Wet Steam
ν = X • νg + (1 - X) • νf
where:
X = Dryness (% / 100)
νf = Specific Volume of Saturated Water
νg = Specific Volume of Saturated Steam
Specific Enthalpy (h) of Wet Steam
h = hf + X • hfg
where:
X = Dryness (% / 100)
hf = Specific Enthalpy of Saturated Water
hfg = Specific Enthalpy of Saturated Steam - Specific Enthalpy of Saturated Water
Specific Entropy (s) of Wet Steam
s = sf + X • sfg
where:
X = Dryness (% / 100)
sf = Specific Entropy of Saturated Water
sfg = Specific Entropy of Saturated Steam - Specific Entropy of Saturated Water
The wetter the steam, the lower the specific volume, enthalpy, and entropy will be because the dryness percentage is a factor of the 100% condition. Since steam dryness has a significant effect on all these values, to enable greater heating efficiency it is crucial to supply steam that is as close to being 100% dry as possible.
| The Relationship Between Steam Dryness and Enthalpy |
|---|
|
| As the amount of water in steam increases, the latent heat decreases, providing less heat to transfer from the steam to the process / product being heated. |
Steam Dryness Decreases During Transport
During transport, radiant heat loss from piping causes part of the steam to lose some of its latent heat and revert back to water, thereby decreasing steam dryness.
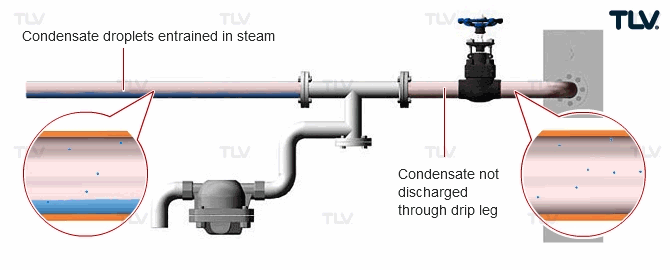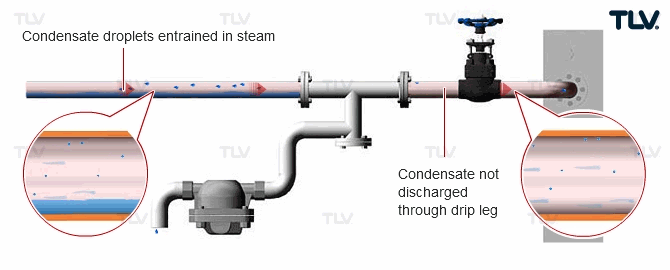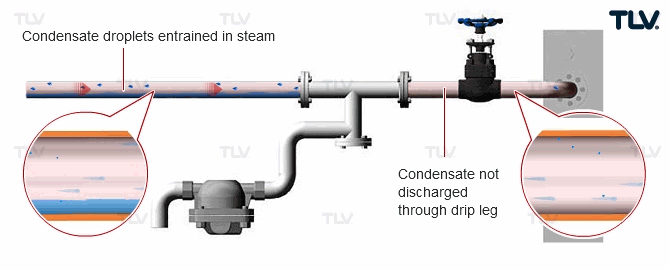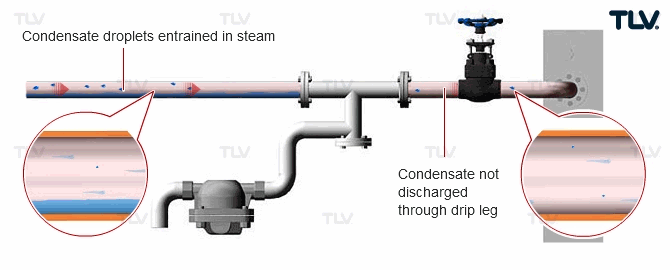
| Water Droplets Entrained in Steam |
|---|
|
| Proper measures should be taken to discharge all condensate within steam piping, including water droplets entrained within the flow of steam. |
Since wet steam not only affects heat transfer efficiency, but can also cause erosion of piping and critical equipment such as turbine blades, it is highly recommended to take preventative measures such as using a steam separator to remove the entrained condensate and by following the advice written in these articles:
Tip
Can steam dryness rise above 100%? It might seem unlikely, but actually it can. When steam is more than 100% dry it is called superheated steam. This type of steam is created by adding heat above the saturated steam threshold. The added heat raises the steam’s temperature higher than its saturation point, allowing the amount of superheat to be easily determined by simply measuring its temperature.