- Home
- Steam Resources
- Steam Theory
- Steam Heating Mechanism
Basics of Steam
Steam Heating Mechanism
In this article, we will first take a look at how steam provides even and rapid heating. This will be followed by a discussion about the heat transfer rate supported by experimental data comparing hot water and steam.
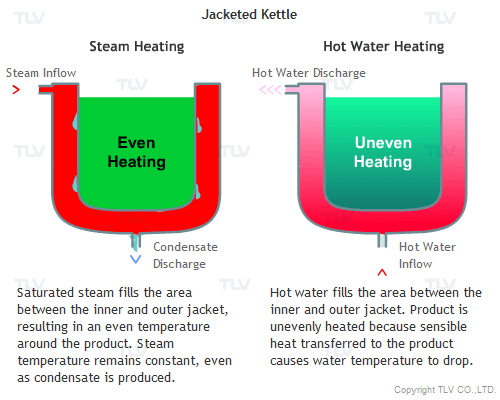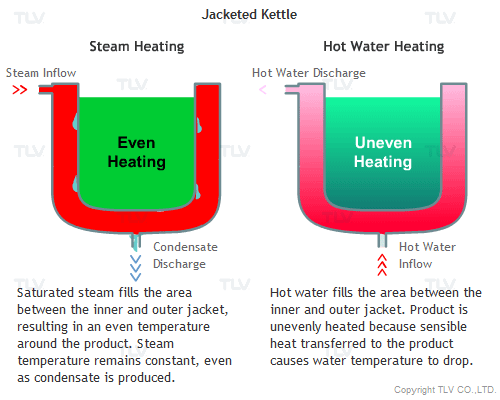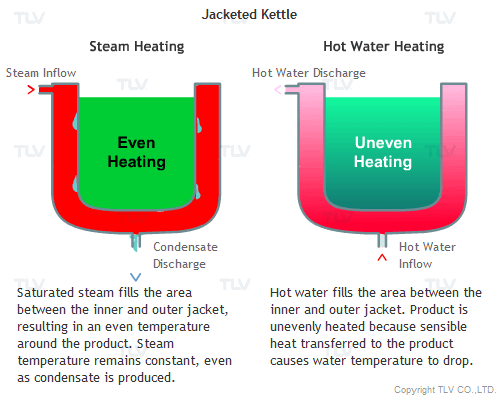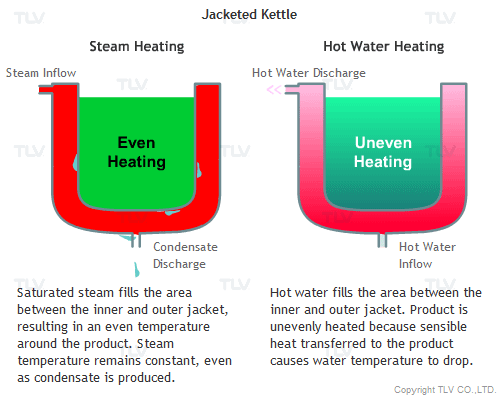
How Does Steam Provide Stable, Even Heating?
Unlike heat transfer by convection (e.g. hot water), heat transfer by condensation (e.g. steam) does not involve a temperature change. When steam condenses on the heat transfer surface, it passes on its latent heat to the product. The condensate then formed still contains its sensible heat, so it is of the same temperature as the steam from which it was produced. This enables even heating across the whole heat transfer surface.
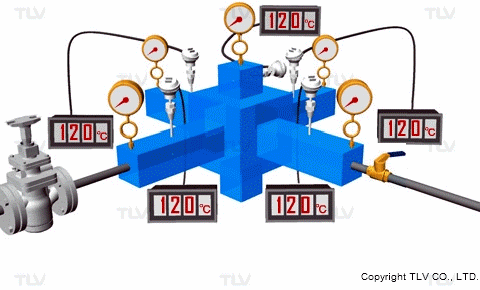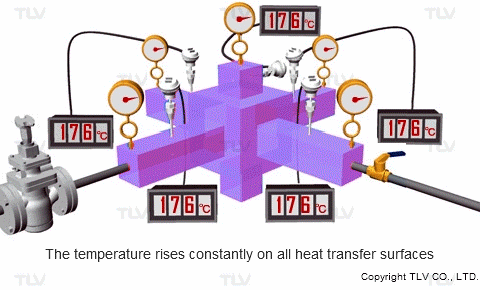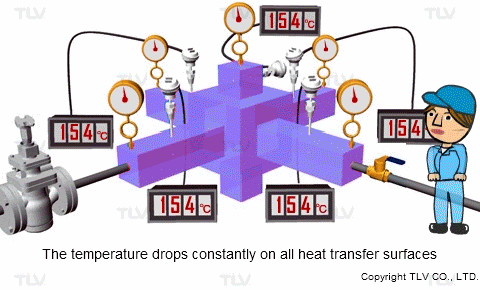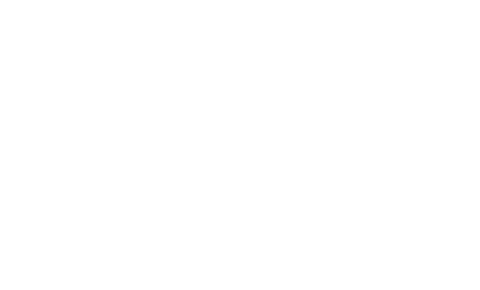
If the pressure at the heat transfer surface (e.g. jacket, shell or coil) of the equipment is held constant, continuous heating at a constant temperature can take place throughout every part of the heat transfer surface.
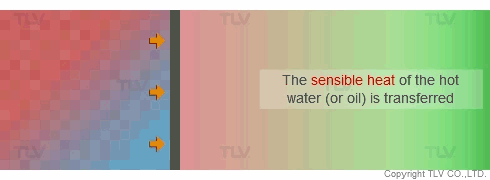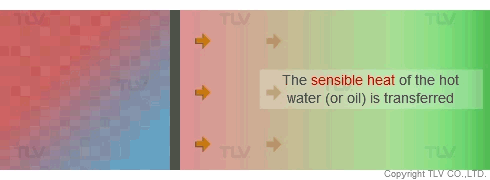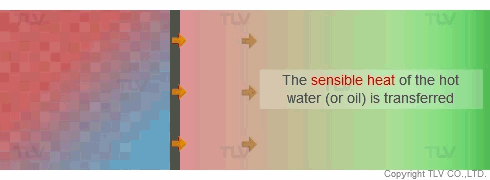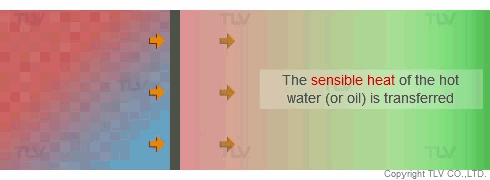
On the other hand, with hot water or oil heating, the temperature of the heating medium is reduced as sensible heat is transferred from the heating medium to the product. The temperature gradient is therefore constantly dropping because each unit of heat transferred will also lower the heating medium's temperature. This can result in uneven heating, which may adversely affect the product being heated.
How Does Steam Provide Rapid Heating?
Heat Transfer from Condensation (Steam)
The secret is in the transfer of heat resulting from the process of condensation.
The latent heat contained in steam is released the instant steam condenses into the liquid state. The amount of latent heat released is 2 to 5 times greater than the amount of sensible heat available from hot water (saturated water) after condensation. This latent heat is released instantaneously and is transferred through the heat transfer surface to the product being heated.

Heat Transfer by Convection (Hot Water and Oil)
In contrast, hot water and oil transfer heat by convective heating, which does not involve a change of state. If left to natural convection, heat transfer is extremely slow. Thus, a pump is typically used to create flow against the heat transfer surface to increase the rate of heat transfer. This is known as forced convection heating.
Heat Transfer Rate
The rate of heat transfer between a moving fluid and a solid is usually indicated by the convective heat transfer coefficient. Its values are roughly considered to be:
- 1000 — 6000 W/(m2°C) [176-1057 Btu/(hr-ft2°F)] for hot water, and
- 6000 — 15000 W/(m2°C) [1057 - 2641 Btu/(hr-ft2°F)] for steam.
In a heat exchanger, however, the heat transfer process cannot be summarized by the convective heat transfer coefficient alone since heat transfer occurs through several mediums. It is thus a combination of the following three mechanisms:
- heat transfer from the heating medium to the surface of the heat exchanger
- heat transfer within the walls of the heat exchanger, and
- heat transfer from the wall surface of the heat exchanger to the product being heated.
Evaluating heat transfer in a heat exchanger therefore requires the overall heat transfer coefficient (i.e. U-value), which takes into account all three mechanisms. Its units are the same: W/(m2°C), or Btu/(hr-ft2°F).
Experimental Data
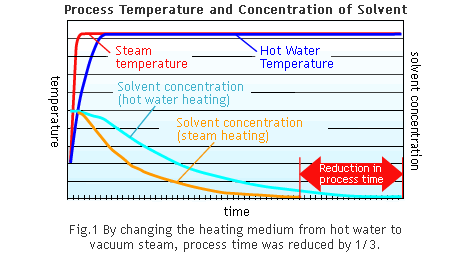
The U-value can vary greatly from one heat exchanger application to another, but experimental data has shown that steam heating can reach U-values up to 1.7 times those of hot water heating. Here is an example of the improvements involved in changing the heating medium of a jacketed kettle from hot water to vacuum steam at company A:
Task: Improve production rate by reducing process time needed to concentrate a chemical agent diluted in a solvent.
Sector: Fine Chemicals
Equipment: Glass-lined Jacketed Kettle (10m3)
| Convective Heat Transfer Coefficient | Overall Heat Transfer Coefficient | Process Time | |
|---|---|---|---|
| Hot Water | 500 W/m2°C [88.1 Btu/(hr-ft2°F)] | 213 W/m2°C [37.5 Btu/(hr-ft2°F)] | 10 h |
| Vacuum Steam | 10000 W/m2°C [1761 Btu/(hr-ft2°F)] | 356 W/m2°C [62.7 Btu/(hr-ft2°F)] | 7 h |
As the data illustrates, the U-value increased by a factor of 1.7, which significantly reduced production time.
For more detailed calculations and equations related to the Overall Heat Transfer Coefficient, read the article here.

